FOR SPONORS & CROs
Small Site.
Big Integrity.
Trusted Data.
Partnering with Kalo Clinical Research ensures access to diverse populations without compromising quality.
We deliver high-integrity, audit-ready data with meticulous care and transparency.
Our operations are lean, our systems are proven, and our team is deeply rooted in the communities we serve—making us an ideal site partner for sponsors and CROs who value precision and purpose.


Main Entrance
A welcoming main entrance, lobby, and waiting area designed to put every guest at ease from the moment they arrive.

4133 Pioneer Parkway Suite 110, West Valley City, UT 84120



4133 Pioneer Parkway Suite 110, West Valley City, UT 84120




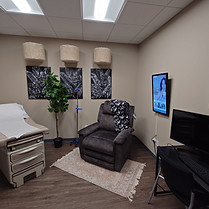
Exam Rooms
Four thoughtfully designed exam rooms that blend comfort with the highest standards of clinical trial excellence.
Lab + Break Room
A dedicated space where precision meets purpose, supporting vital research with meticulous lab work.




What Sponsors
Say Sets Kalo Apart
WHAT MAKES US DIFFERENT

✓ Strong protocol adherence and PI engagement
✓ Clean, monitor-approved data
✓ High retention from participant-first engagement
✓ Access to underrepresented populations in Utah
✓ Rapid enrollment and responsive communication
✓ Values-led site operations built for scale
“You get the gold-standard systems of a top-tier research site, led with humanity, built on connection. That’s what sets us apart.”
Diversity is
Our Standard
We Are Here to Serve Our Diverse Communities

Kalo is based in West Valley City, Utah—home to the third-largest Pacific Islander population in the U.S., alongside vibrant Hispanic, Native American, and other underrepresented communities.
Our studies are designed with accessibility and cultural intelligence at the forefront—building trust from day one.
Our current capabilities include studies in:
-
Internal Medicine
-
Cardiology
-
Dermatology
-
Pulmonology
-
Metabolic Conditions
Including diabetes & obesity

OUR SITE STRENGTHS
Built for CRO Alignment and Quality Outcomes
Whether you're a CRO or a direct sponsor, we’d love to talk about your protocol needs and how we can support your study with integrity, agility, and inclusion.
You Need Sites That Can Deliver. We're Ready.
25+
Principal Investigators with 25+ years of academic + clinical research leadership
SOPs
Structured SOPs + ready-to-integrate systems
A+
Clean data, on time, every time
e+
eSource and eRegulatory systems built for remote monitoring
100%
Dedicated study coordinators and medical oversight
5*
Five star community-trusted relationships → faster enrollment and quality retention




